how does a car battery work chemistry
The electrolyte carries positively charged lithium ions from the anode to the cathode and vice versa through the separator. The Chemistry of How A Car Battery Works The chemical solution Sulfuric acid given as H2SO4 which is a compound of hydrogen sulfur and oxygen The cathode lead dioxide given by the chemical annotation PbO2 a.
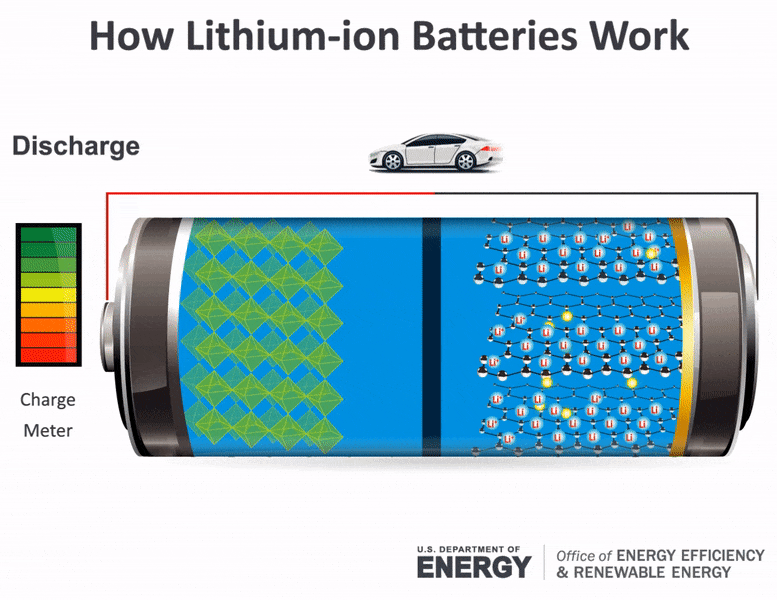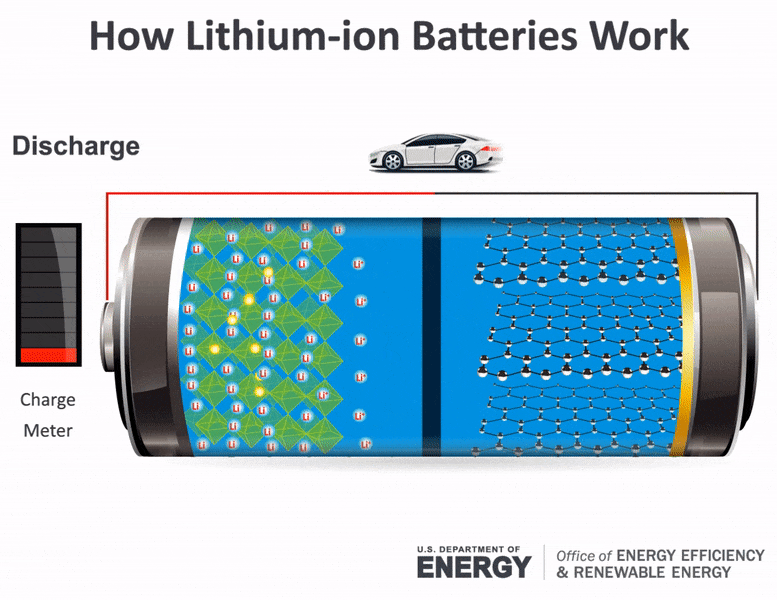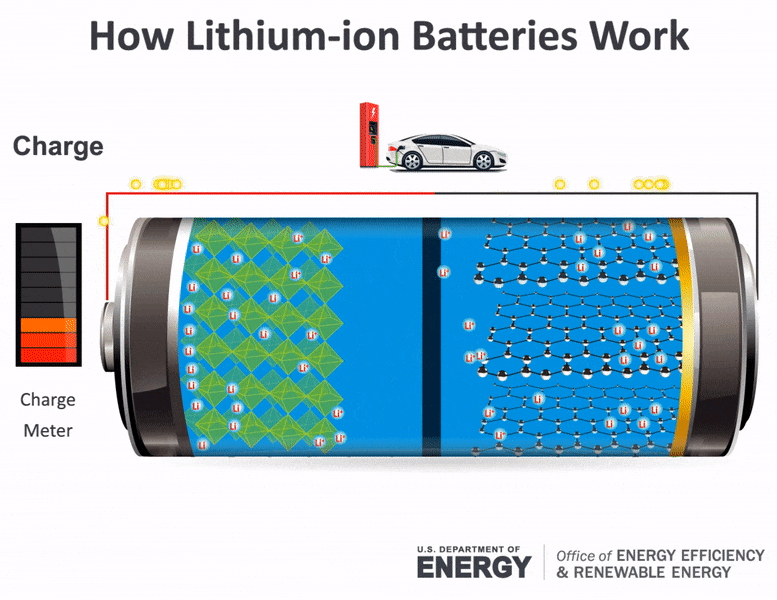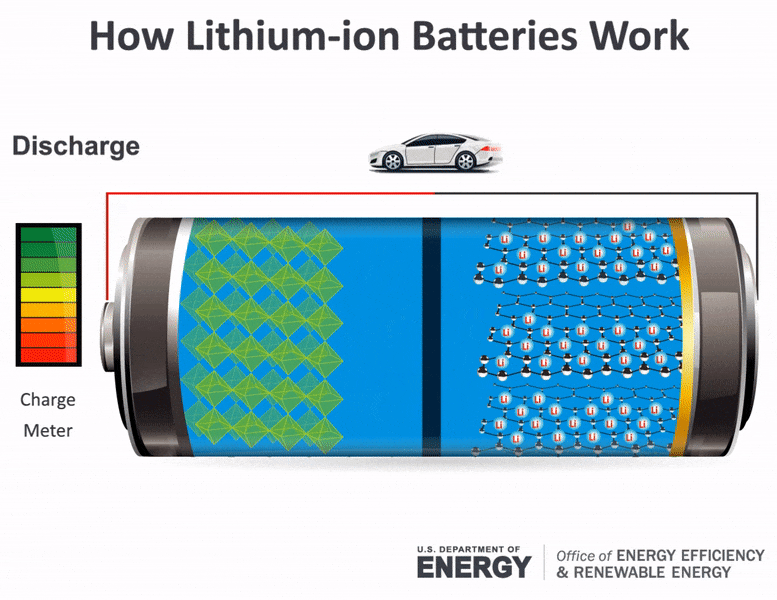
Science Made Simple What Are Batteries And How Do They Work
The movement of the lithium ions creates free electrons in the.
. Inside the battery are 3 important things. The best way to understand these reactions is to see them for yourself. Ad Need a New Battery.
In the case of car batteries a fully charged battery will measure about 126 volts between the terminals while a completely dead battery will measure. Battery chem is an american invention that is considered to be a green technology. How does a car battery work learn from the basics where we use and battery and how batteries work.
How does a car battery work learn from the basics where we use and battery and how batteries work. How do car batteries work. Batteries and similar devices accept store and release electricity on demand.
How does a car battery work chemistry. Hydrogen H Oxygen O 2 Lead Pb Sulfur S Connection of an external consumer starts the chemical reaction in the battery. When a device is connected to a battery a light bulb or an electric circuit chemical reactions occur on the electrodes that create a flow of electrical energy to the device.
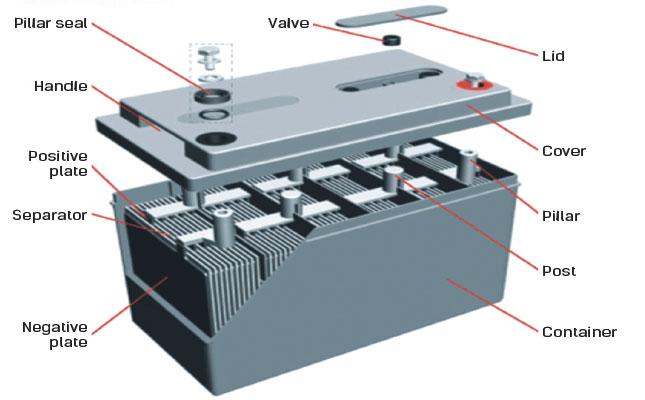
SLI stands for starting lighting and ignition This type of battery provides short bursts of energy in order to power your lights accessories and engine. Chemical reactions inside the car battery. A car battery is actually 6 smaller batteries that are lined up in series.
In this electro-chemical process four materials react with each other. When the battery is in use lead oxide in the cathode reacts with sulphuric acid to form lead sulfate PbSO4. Most car batteries rely on a lead-acid chemical reaction to get things moving and grooving.
An electrical charge from outside the battery charges it and activates a chemical reaction within the battery. A battery is a device that transforms chemical energy to electric energy and that is the basic way a car battery works. It accomplishes this through the usage of cells which contain and store the energy until needed.
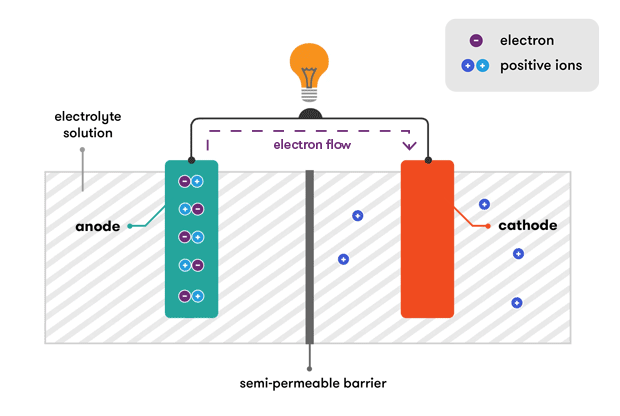
A battery is made up of an anode cathode separator electrolyte and two current collectors positive and negative. With thanks to Squarespace for sponsoring this video. During a discharge of electricity the chemical on the anode releases electrons to the negative terminal and ions in the electrolyte through whats called an oxidation reaction.
This is the chemistry used in a typical car battery. Cathodes anodes and solutions. How A Car Battery Works.
Battery consists of three main components. There are three components within the battery namely two connectors known as an anode and a cathode and then a chemical solution that the connectors sit in. The cathode is a positive plate made of PbO2 while the.
Lead-acid battery rechargeable. Most standard car batteries contain six cells that are situated in a row inside the plastic casing. There are 2 connectors that go out of the battery.
For example logs store energy in their chemical bonds until burning converts the energy to heat. These batteries fall into the SLI category. The lithium ions move back from the cathode to the anode.
The chemical energy results from the lead-acid reaction that begins the whole process. Batteries use chemistry in the form of chemical potential to store energy just like many other everyday energy sources. Once the battery jolts the engine to life.
As such the car battery is categorised as a. Every battery is made up of three parts. Get Back on the Road with a Proven Tough Duralast Battery.
The general way that a battery works is that when an electronic circuit is connected to the battery electrons are allowed to flow. The anode and cathode store the lithium. A car battery uses lead-acid technology to turn chemical energy into electricity.
The chemical components of a car battery also known as a lead-acid battery are lead dioxide PbO2 in the cathode lead Pb in the anode and the solution is sulphuric acid H2SO4. The electrodes are usually made of lead dioxide and metallic lead while the electrolyte is a sulfuric acid solution. This causes the voltages of each battery to add.
The electrolyte a mixture of sulfuric acid H 2 SO 4 and. AutoZone Has You Covered at Americas 1 Battery Destination. An anode electrolyte and a cathode.
For clarity I will briefly discuss how the battery works from the start. A car battery stores energy in chemical form and converts it into electrical energy.
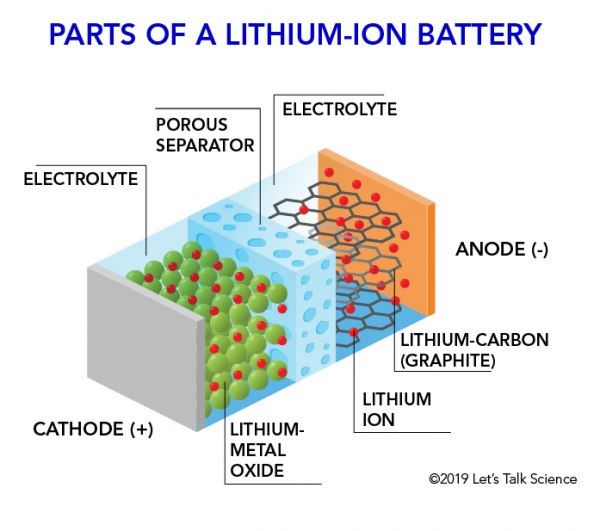
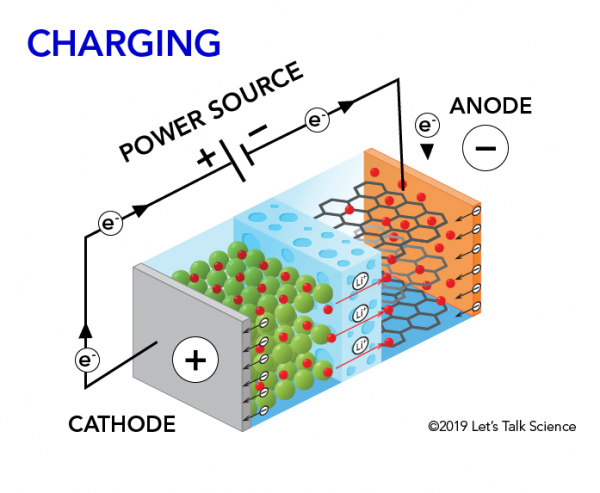
How Does A Lithium Ion Battery Work Let S Talk Science
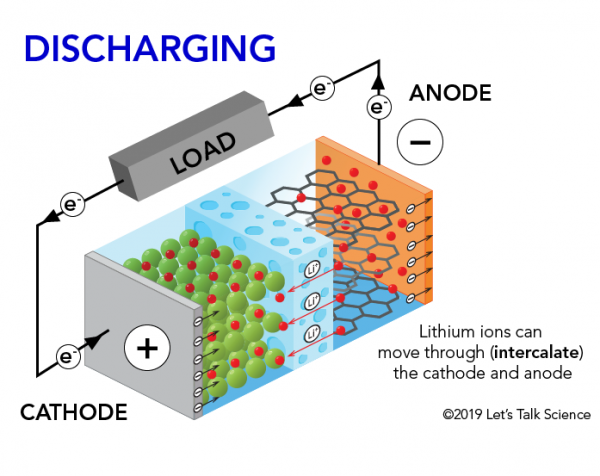
How Does A Lithium Ion Battery Work Let S Talk Science
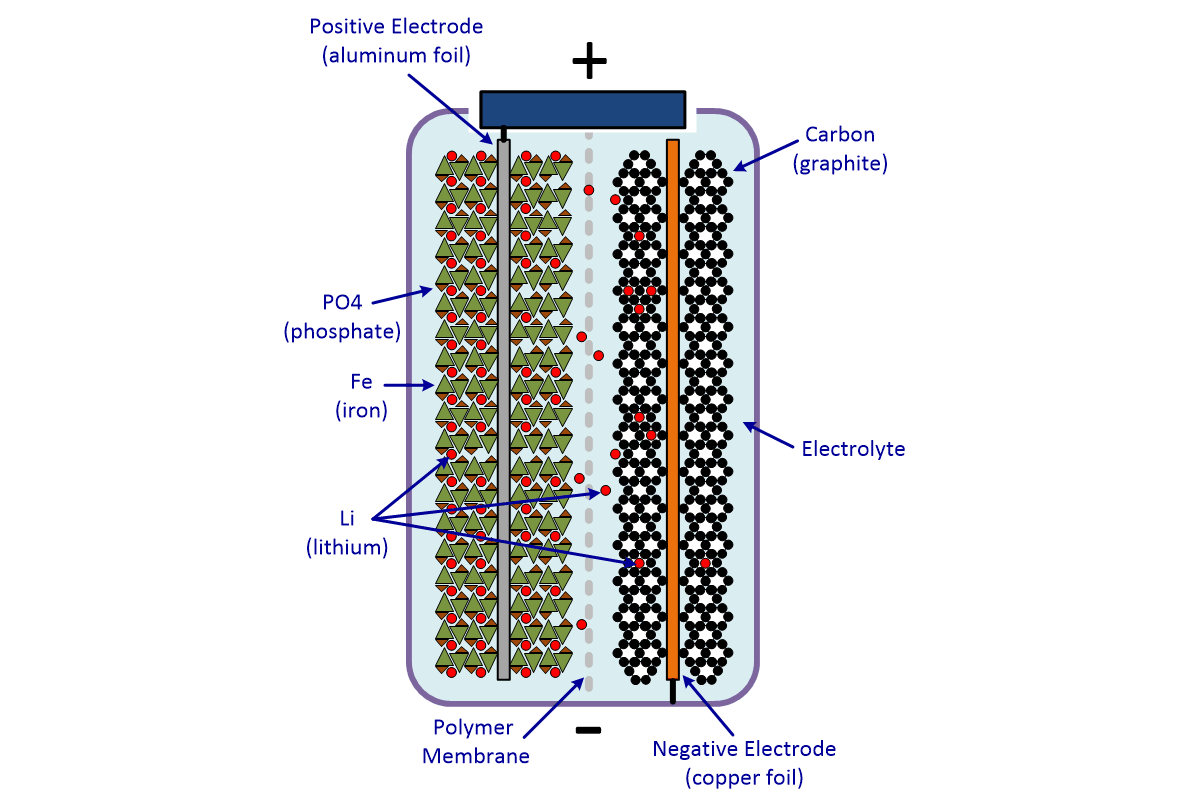
Electric Car Battery Chemistry What S The Difference Carexpert
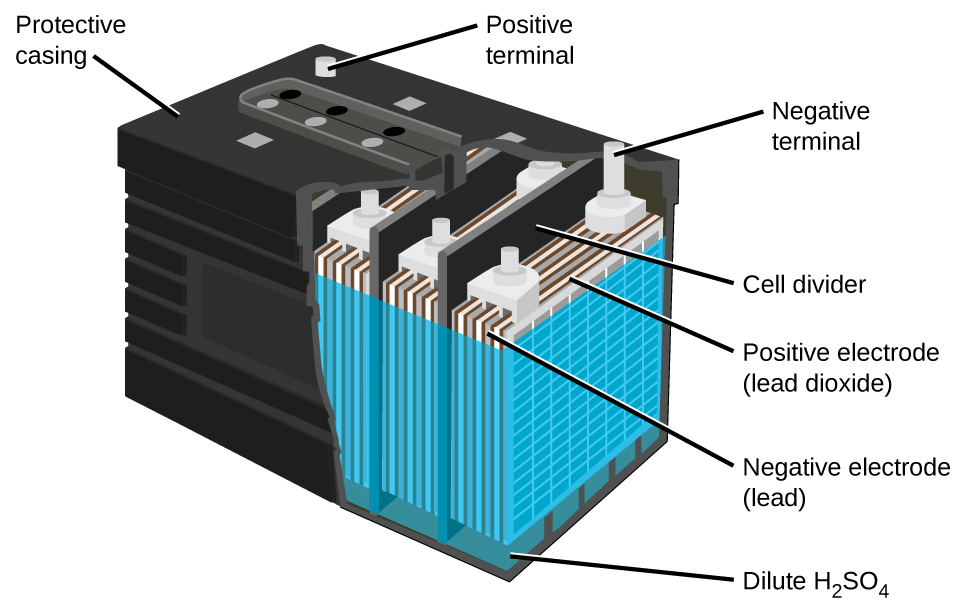
17 5 Batteries And Fuel Cells Chemistry
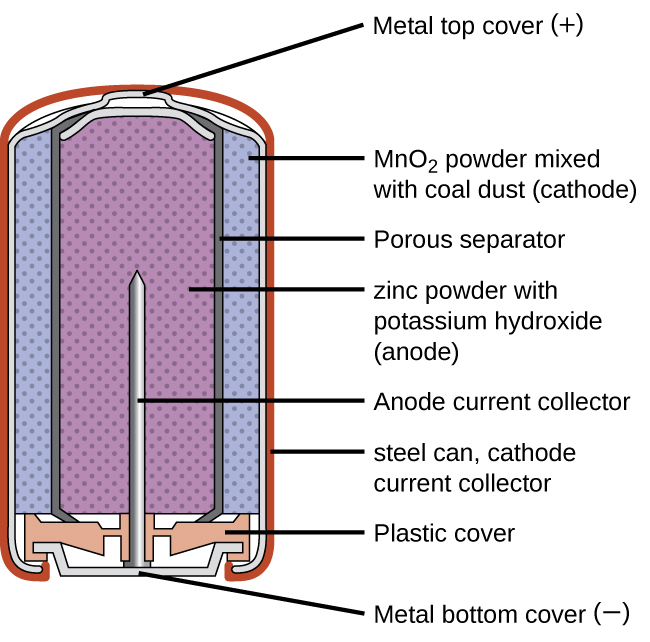
17 5 Batteries And Fuel Cells Chemistry

How Does A Lithium Ion Battery Work Let S Talk Science

Working Of Car Batteries Askiitians Blog One Place For All Updates On Iit Jee Medical Exams
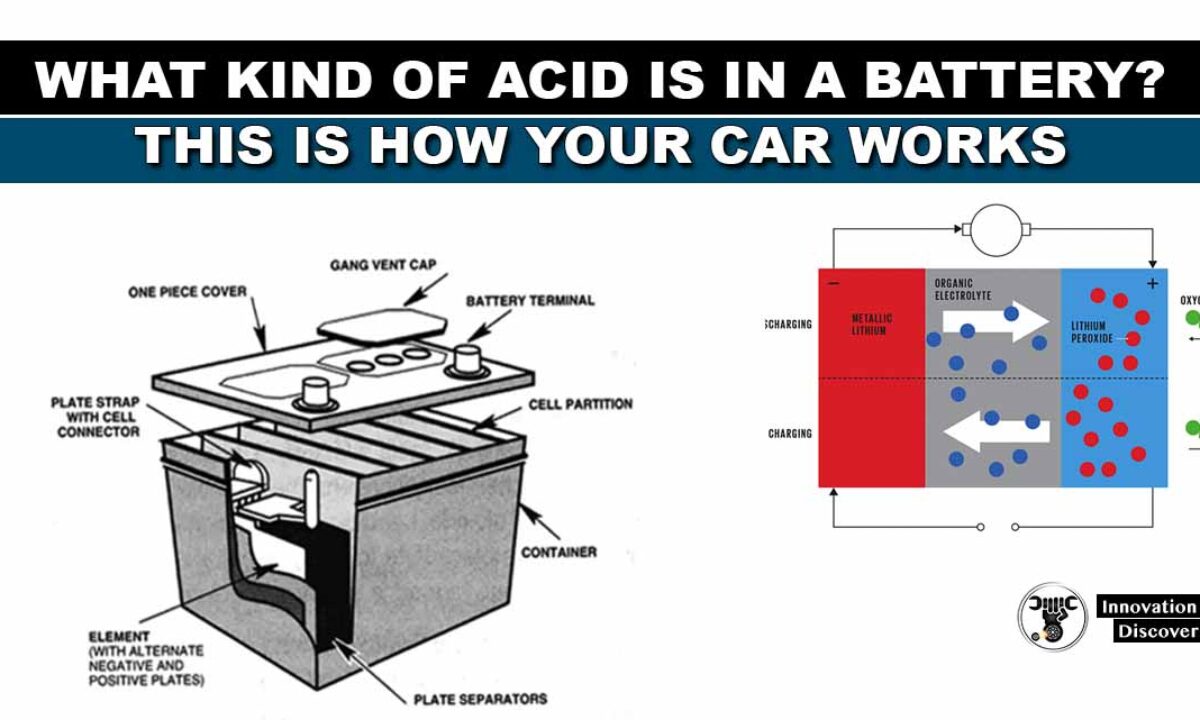
What Kind Of Acid Is In A Battery This Is How Your Car Works
How Does A Car Battery Work Mach 1 Services

How A Car Battery Works The Engineering Mindset

How A Car Battery Works The Engineering Mindset

How Much Does A Replacement Car Battery Cost
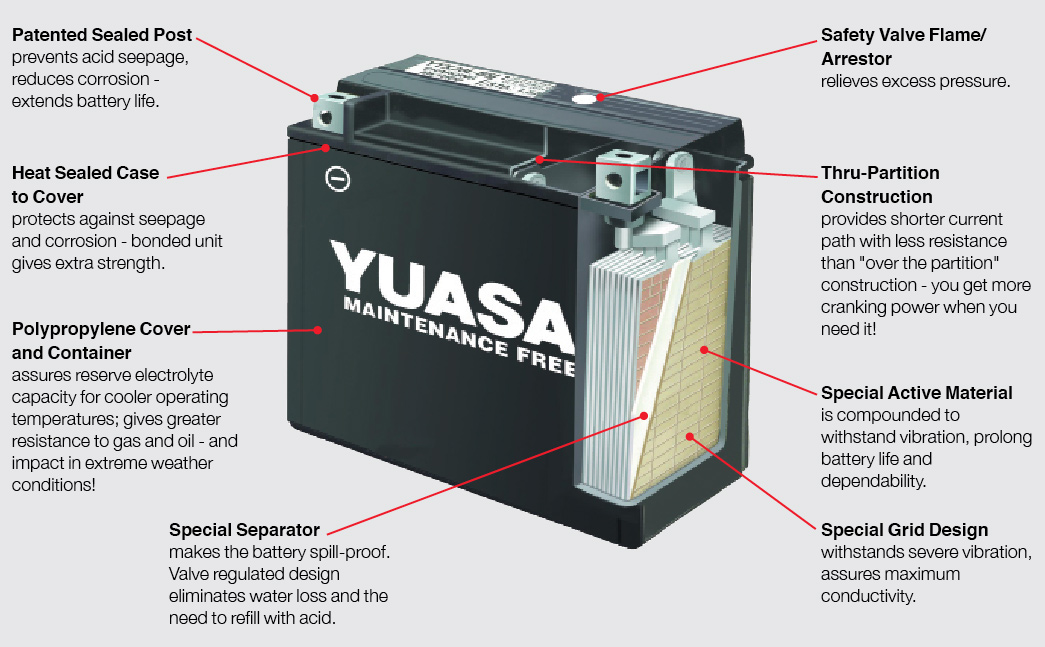
How A Battery Works Information Guide From Yuasa

Battery 101 How Does A Car Battery Work Firestone Complete Auto Care

How A Car Battery Works The Engineering Mindset

How A Car Battery Works The Engineering Mindset
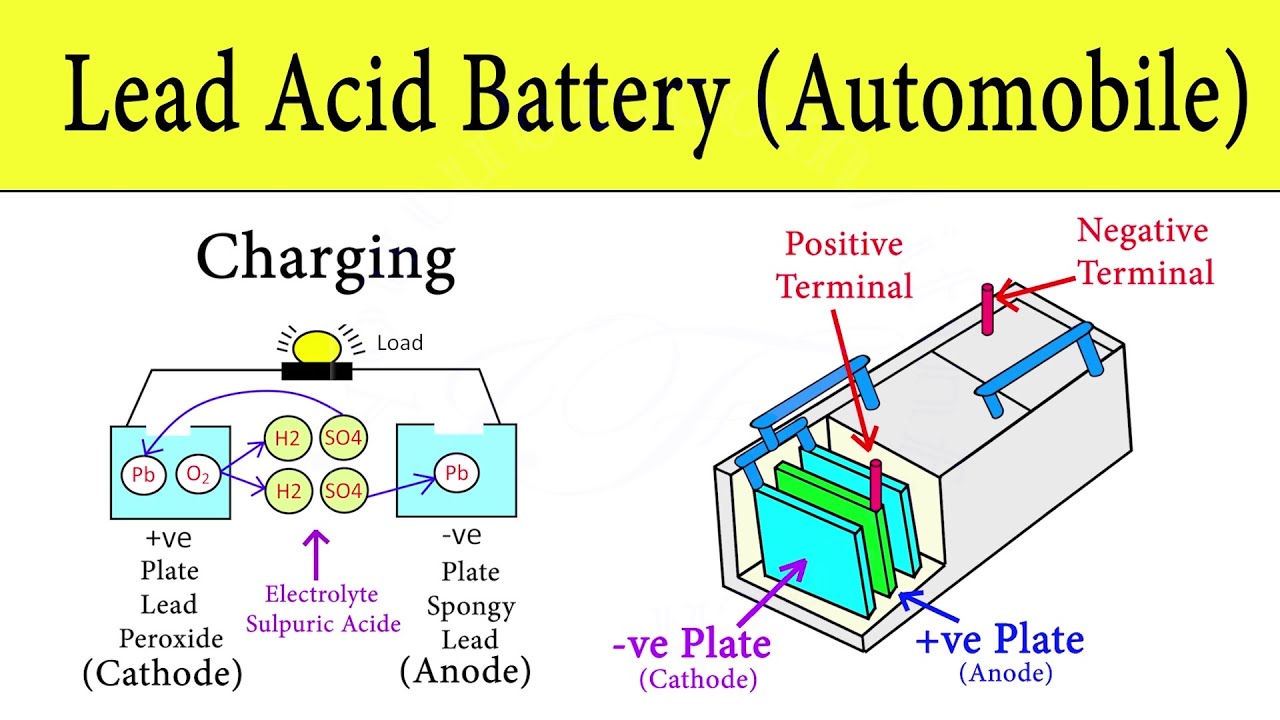
Lead Acid Battery How Car Battery Works Automobile Battery Working Principle Animation Youtube